A 2022 Parkinson’s Foundation-backed study revealed that nearly 90,000 people are diagnosed with Parkinson’s disease (PD) in the U.S. each year. This represents a steep 50% increase from the previously estimated rate of 60,000 diagnosed annually. Rates of PD are expected to double by 2040.
PD incidence rates are higher in certain geographic regions: the “Rust Belt” (parts of the northwestern and midwestern U.S. previously regulated by industrial manufacturing), Southern California, Southeastern Texas, Central Pennsylvania and Florida.https://www.parkinson.org/about-us/news/incidence-2022
Parkinson’s is described as an age-related degenerative brain disorder. The disorder causes uncontrollable movements such as shaking, stiffness, loss of balance and loss of coordination. Symptoms begin gradually and worsen over time. With progression people may have tremors in the hands, arms, legs, jaw or head, muscle stiffness, slowness of movement and impaired balance and lack of coordination that often leads to falls.
Patients may also have mental and behavioral changes, sleep problems, depression, memory difficulty, fatigue, and difficulty swallowing, chewing and speaking. Facial expression lacks animation. Patients develop a Parkinsonian gait with a tendency to lean forward, take small quick steps with little arm swinging. Symptoms often begin subtly on one side of the body. As the disease progresses symptoms intensify and involve both sides of the body. Some people with PD may experience changes in their cognitive function, including problems with memory, attention, and the ability to plan and accomplish tasks. These changes may progress to symptoms of dementia.
Men are usually affected more than women. The age of onset is after age 50 years, or earlier in the case of the genetic form of the disease. Some forms of the disease are linked to specific genes (LRRK-2, a-synuclein, Pink-1, Parkin, GBA, UCHL-1, PARK-7).
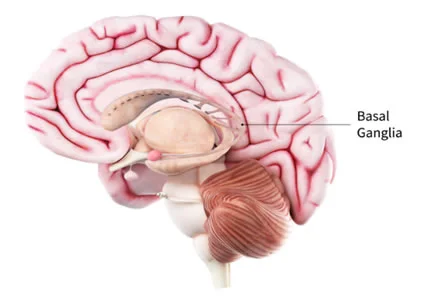
The pathological cause of Parkinson’s is impairment or death of nerve cells in the basal ganglia. This is the area of the brain that controls movement. Basal ganglion cells produce the neurotransmitter known as dopamine. The loss of dopamine causes motor problems that are commonly associated with the disease. Nerve cells also lose the capacity to produce norepinephrine, the neurotransmitter that controls many of the sympathetic functions in the body such as heart rate and blood pressure.
Loss of norepinephrine is thought to explain some of the non-movement symptoms of PD such as fatigue, irregular blood pressure, decreased gastrointestinal motility and autonomic dysregulation (sudden drop in BP when rising to a standing position).
In PD many brain cells contain Lewy bodies which are unusual clumps of the highly charged protein, alpha-synuclein. The functions of this protein are not well understood. Alpha synuclein is found at the synaptic terminals of neurons so it is thought to play a role in transmission of chemical signaling, especially regarding dopamine via modulation of its precursor, tyrosine.
Holistic Perspectives
The holistic approach seeks to identify the root causation of disease. Bredeson notes that when mitophagy (the degradation of mitochondria by autophagy) is blocked in mice, PD develops. According to a study in Parkinson’s Related Disorders, (Ekstrand, 2009) genes that cause familial PD as well as toxins cause mitochondrial dysfunction in dopamine neurons. To test this hypothesis researchers developed a mouse model of PD that they termed “MitoPark.” They inactivated a protein essential for mitochondrial DNA function, thus depleting dopamine in these neurons. The MitoPark mice developed features of PD.
There is increasing recognition of the role environmental toxins play in the development of PD. A growing body of evidence suggests that exposure to pesticides, solvents, heavy metals, micro plastics and air pollution, is at least in part responsible for this rapid growth in rates of PD (Shan, 2023). Trichloroethylene, a chemical commonly used in dry cleaning, carpet cleaners, shoe polish, and industrial processes has been pinpointed by several researchers as a significant contributor.
Treatment
There are many medications available to treat PD but, to date, none have proven effective in slowing or stopping the disease process. However, some newer approaches are showing promise.
- High dose thiamine (B1), given orally or intravenously have been shown to improve the motor symptoms associated with PD. It is hypothesized that a severe focal thiamine deficiency due to a dysfunction of thiamine metabolism could cause damage to the dopamine neurons (Costantini, 2013).
- Specific gut bacterial profiles have an association with PD. Studies have found imbalances in bacteria that produce short chain fatty acids in people with PD. Due to these imbalances, there is potential for impairment of the gut intestinal barrier and the blood-brain barrier resulting in an increase in neural inflammation (Li, 2023).
Based on these new understandings, there are new approaches that offer promise. Some functional practitioners feel thattreatment for PD should include detoxification from heavy metals and environmental toxins along with glutathione support, provision of nutritional support for mitochondria (ubiquinol, PQQ, NAD), and to decrease inflammation (NLRP3 inhibition, curcumin, probiotics, EGCG, quercetin, resveratrol), increase glial derived neurotrophic factor (Ashwagandha, EGCG, Rehmannia [Chinese Foxglove]), rebalance the gut microbiome, optimize immune function, and balance hormones.
References
Costantini, A., Pala, M. I., Compagnoni, L., &Colangeli, M. (2013). High-dose thiamine as initial treatment for Parkinson’s disease. BMJ case reports, 2013, bcr2013009289. https://doi.org/10.1136/bcr-2013-009289
Ekstrand, M. I., & Galter, D. (2009). The MitoPark Mouse – an animal model of Parkinson’s disease with impaired respiratory chain function in dopamine neurons. Parkinsonism &Related Disorders, 15 Suppl 3, S185–S188. https://doi.org/10.1016/S1353-8020(09)70811-9
Li, Z., Liang, H., Hu, Y., et al. (2023). Gut bacterial profiles in Parkinson’s disease: A systematic review. CNS neuroscience & therapeutics, 29(1), 140–157. https://doi.org/10.1111/cns.13990
Shan, L., Heusinkveld, H.J., Paul, K.C. et al. Towards improved screening of toxins for Parkinson’s risk. npj Parkinsons Dis. 9, 169 (2023). https://doi.org/10.1038/s41531-023-00615-9